Removing Disease-Causing Proteins from Circulation
It is well established that many circulating and membrane proteins can drive a variety of autoimmune and other diseases. Existing therapeutic approaches are often broad-acting and nonspecific, which can cause off-target side effects. These limitations don’t allow for the fast, complete and deep removal of the offending protein – which is required to significantly improve outcomes. The safety profile and lack of deep efficacy of current approaches, including blocking monoclonal antibodies, relegates them to late-line use. At GlycoEra, we are developing bifunctional biologics with the ability to achieve exquisite precision which enables removal of disease-causing proteins with unprecedented speed and depth while avoiding systemic off-target effects.

GlycoEra Degraders
Bifunctional biologics for extracellular and membrane protein degradation
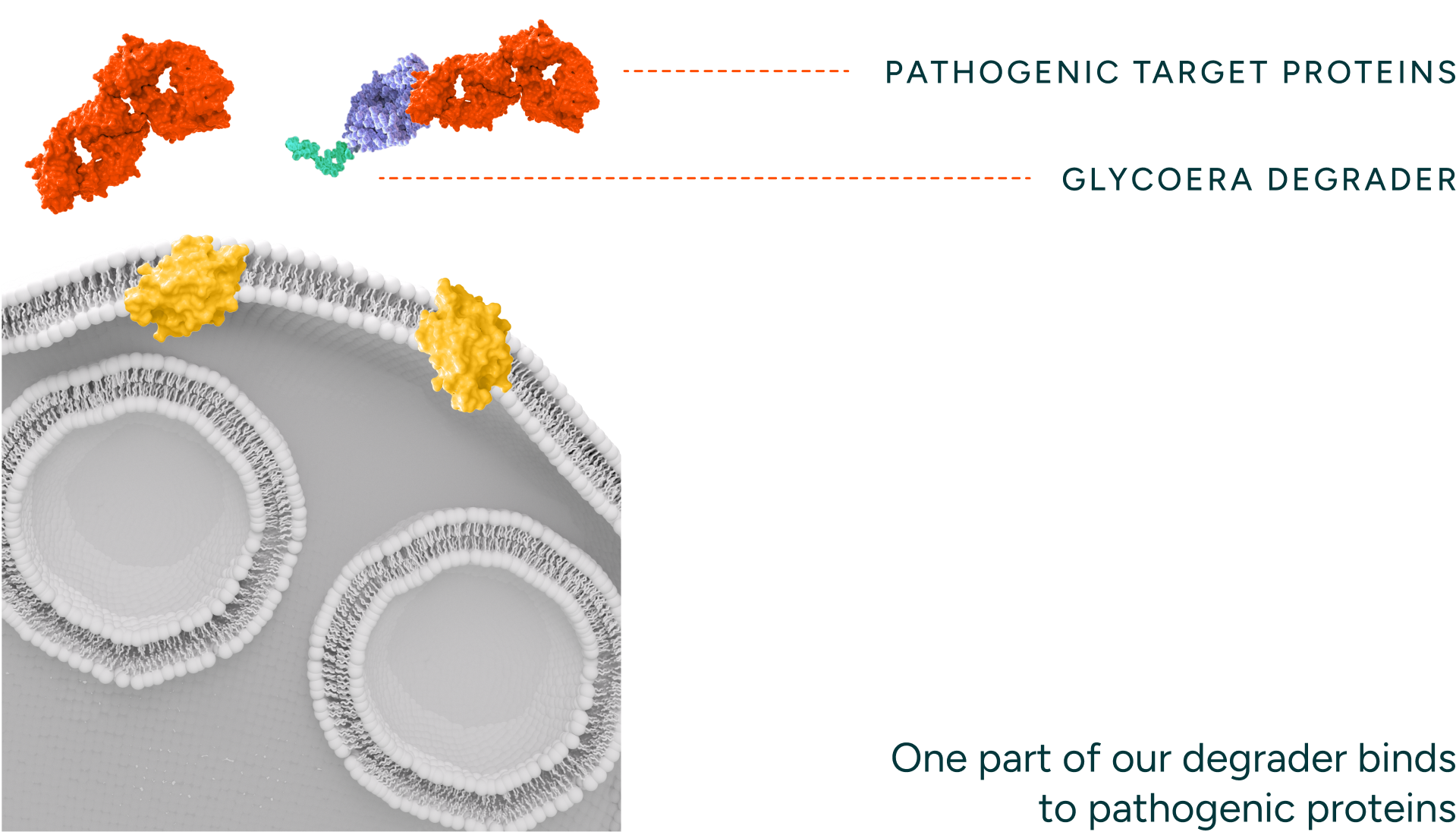
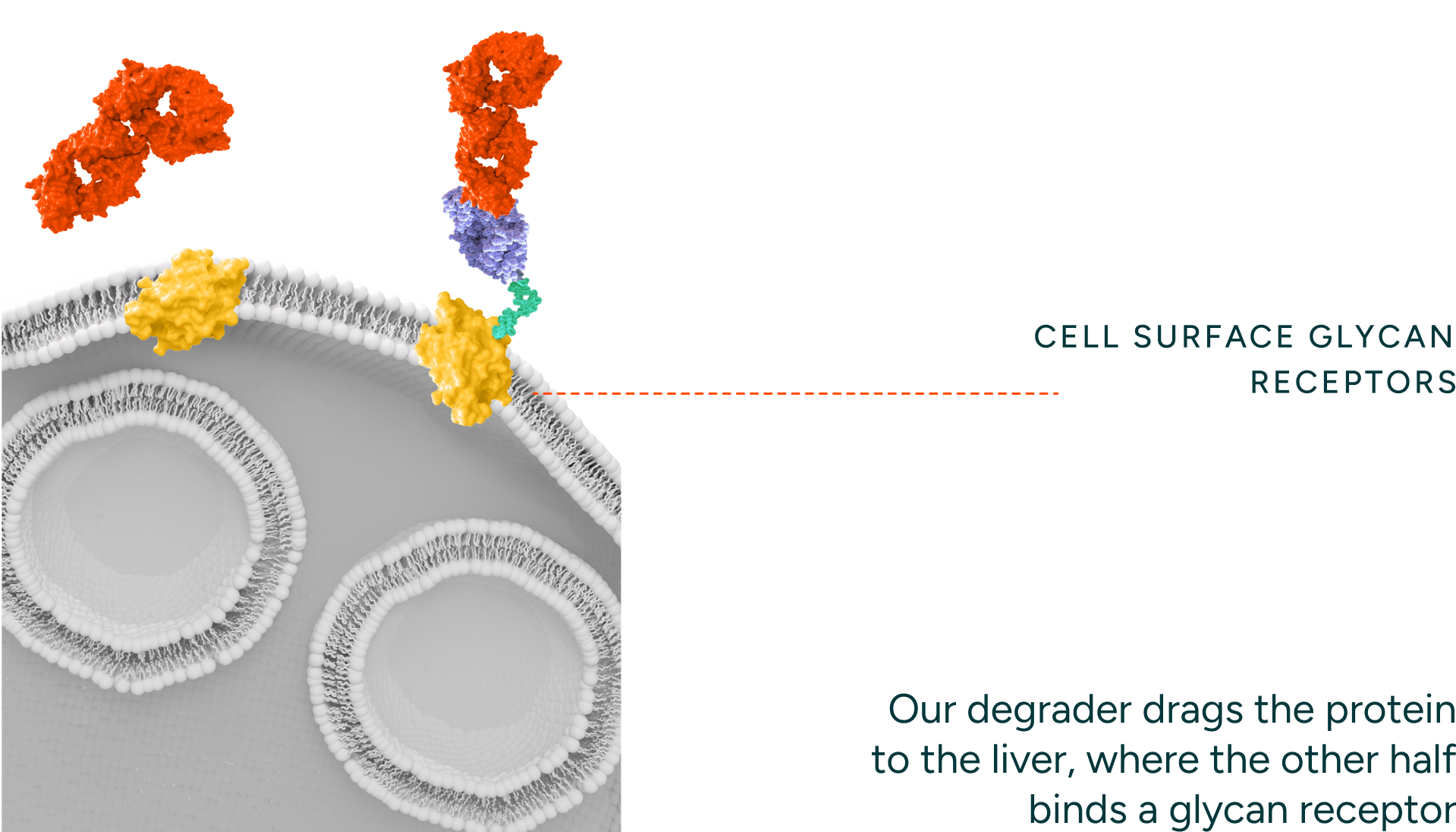
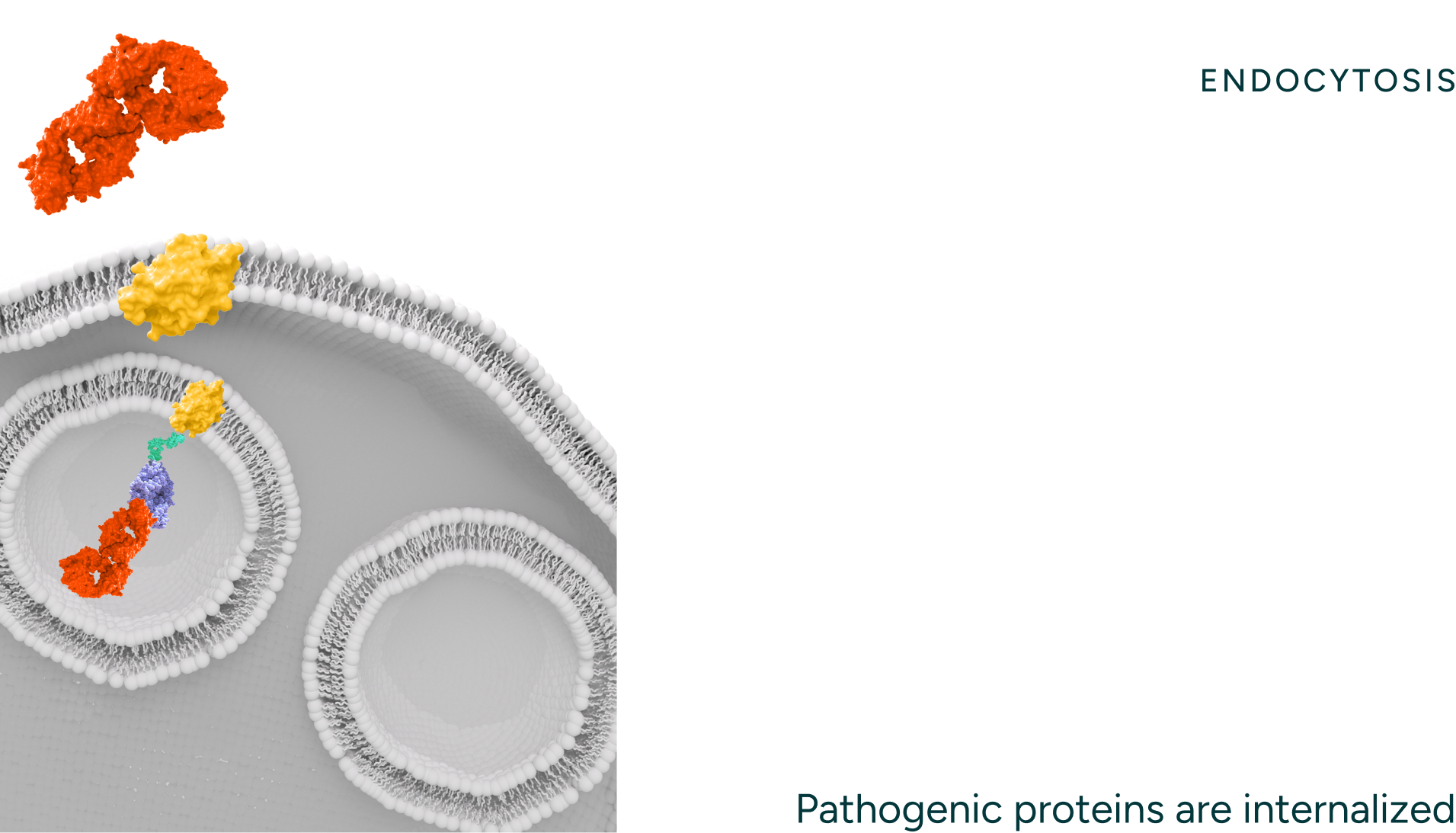
Our degraders bind to the disease-causing protein target and engage endocytic receptors.
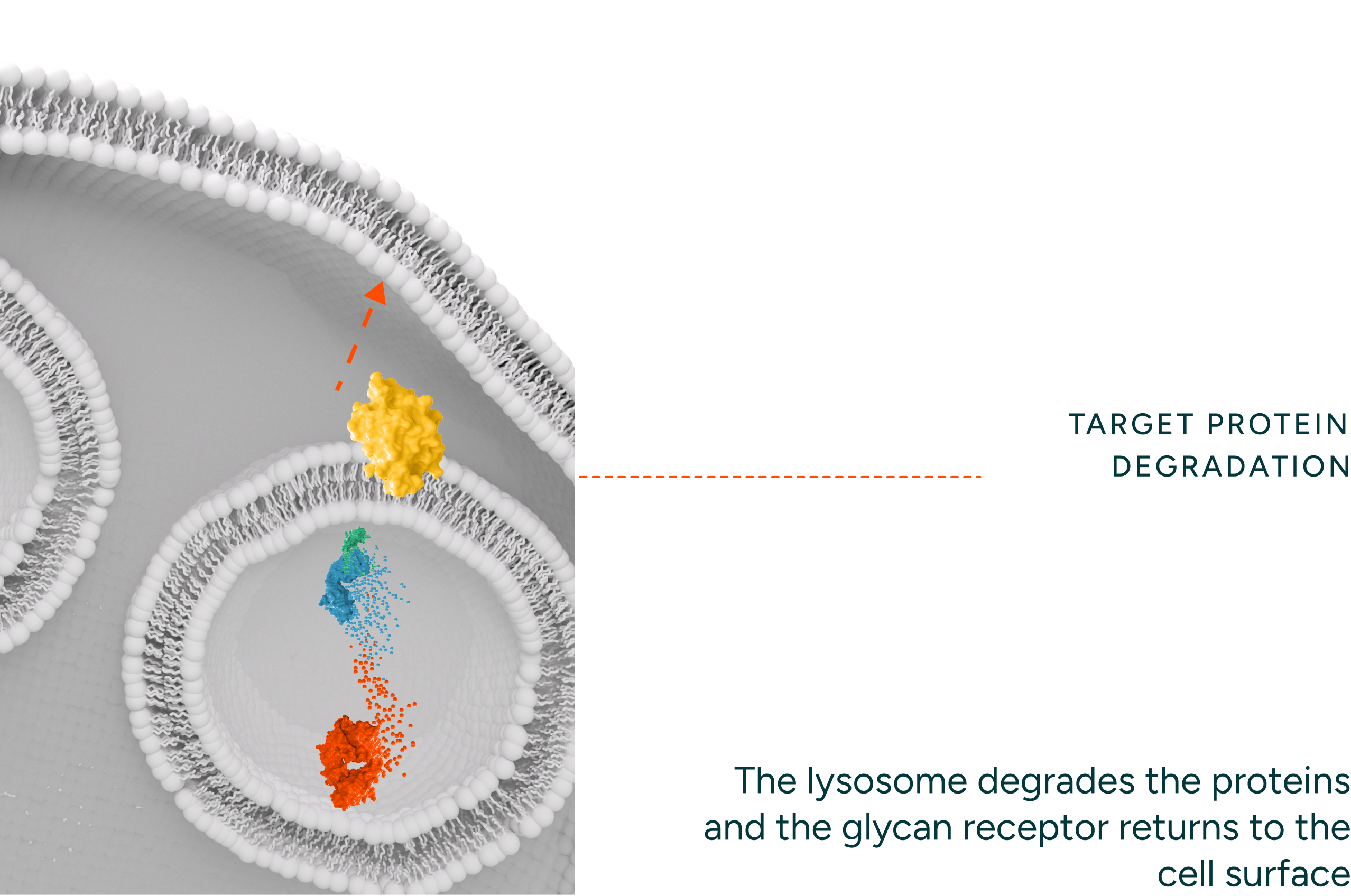
Our degraders are bispecific molecules which leverage a naturally occurring process to degrade proteins. One part of the molecule binds a target protein and the other binds endocytic receptors which traffic the target protein to the lysosome for degradation. Unlike intracellular protein degraders, like PROTACs and molecular glues, our technology can access targets that are extracellular and membrane proteins, thereby opening up novel targets and therapeutic areas for intervention. Our degraders employ novel engager technology that drives optimal binding with the degradation receptors (i.e. ASGPR) enabling rapid internalization and recycling kinetics.
1
2
3
4
Animation of
GlycoEra Degrader
Pathogenic Protein
Glycoera degrader

Glycan receptor
Advantages of our Platform
Simple, one-step manufacturing:
We produce our degraders in our novel expression system in a simple one-step, recombinant process.
Highly reproducible and flexible:
GlycoEra has created a library of cells engineered to express specific glycans on proteins. We have successfully expressed antibodies, Fabs, scFv, Fc fusion and nanobodies with desired homogenous glycans.